

Experiment 15 Chromatography of Amino Acids |
Introduction/Background
Amino acids are the building blocks of proteins. The amino acid sequence determines the structure and function of the protein. Amino acids are also precursors for other biologically important molecules such as some hormones and neurotransmitters. There are 20 biologically important amino acids. They have at least one amino group (-NH2) and one carboxyl group (-COOH). The general structure for an amino acid is:

The side chain(s) ("R" in the general structure) for each amino acid are different. The liver removes excess amino acids in our bodies. Amino acids are found in all foods except oil. Essential amino acids are those that must come from diet. Our bodies are not able to produce these, and they include isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. The rest can be made by us, and are non-essential. See the structure of amino acids.
Key Concepts
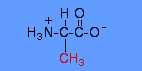 alanine structure |
When an amino acid is dissolved in water, it exists in solution as the dipolar ion, or zwitterion. A zwitterion can act as either an acid or a base and has both a positive and negative charge. Substances having this dual nature are amphoteric and are often called ampholytes. An example of the zwitterionic form of alanine is given. |
| Paper chromatography is a method for analyzing mixtures by separating the components in the mixture by their size and solubility. Remember that amino acids have different 'R' groups. These groups can add to the amino acid's polarity and size. For example alanine, whose 'R' group is a -CH3, is much smaller and less polar than say aspartic acid, whose R group is -CH2COOH. Paper chromatography separates components based on their size and polarity. Molecules that are lighter move further up the paper than heavier molecules. Also, since the solvent is polar, more polar molecules will move farther down the paper. | |
Example 1: Identify the amino acid in a chromatogram by calculating their Rf values.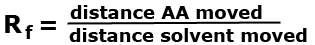  Answer: click to reveal |