Electronic Structure
The content that follows is the substance of General Chemistry Lecture 24. In this lecture we Introduce the concepts of electromagnetic radiation, wavelength, frequency and the Photoelectric Effect.
Electromagnetic Radiation
Electromagnetic waves are produced by the motion of electrically charged particles. These waves are also called "electromagnetic radiation" because they radiate from the electrically charged particles. They travel through empty space as well as through air and other substances.
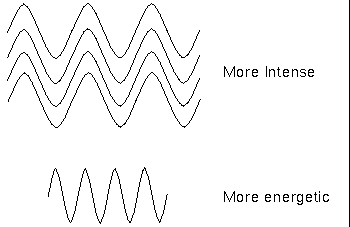
Scientists have observed that electromagnetic radiation has a dual "personality." Besides acting like waves, it acts like a stream of particles (called "photons") that have no mass. The photons with the highest energy correspond to the shortest wavelengths.
There are some general properties shared by all forms of electromagnetic radiation:
- It can travel through empty space. Other types of waves need some sort of medium to move through: water waves need liquid water and sound waves need some gas, liquid, or solid material to be heard.
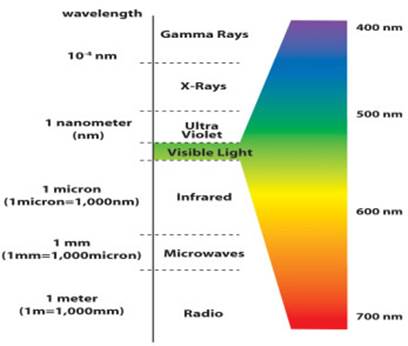
- The speed of light is constant in space. All forms of light have the same speed of 299,800 kilometers/second in space (often abbreviated as c). From highest energy to lowest energy the forms of light are Gamma rays, X-rays, Ultraviolet, Visible, Infrared, Radio. (Microwaves are high-energy radio waves.)
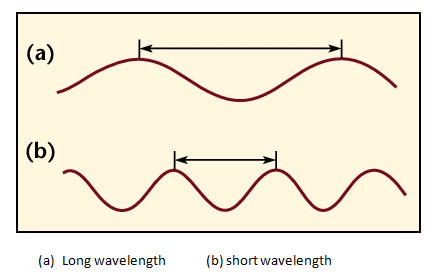
- A wavelength of light is defined similarly to that of water waves---distance between crests or between troughs. Visible light (what your eye detects) has wavelengths 4000-8000 Ångstroms. 1 Ångstrom = 10-10 meter. Visible light is sometimes also measured in nanometers(nm): 1 nanometer = 10-9 meter = 10 Ångstroms, so in nanometers, the visible band is from 400 to 800 nanometers. Radio wavelengths are often measured in centimeters: 1 centimeter = 10-2 meter = 0.01 meter. The abbreviation used for wavelength is the greek letter lambda: λ.
Besides using wavelength to describe the form of light, you can also use the frequency--the number of crests of the wave that pass by a point every second. Frequency is measured in units of hertz (Hz): 1 hertz = 1 wave crest/second. For light there is a simple relation between the speed of light (c), wavelength (λ), and frequency (v):
v = c/λ.
Since the wavelength λ is in the bottom of the fraction, the frequency is inversely proportional to the wavelength. This means that light with a smaller wavelength has a higher (larger) frequency. Light with a longer wavelength has a lower (smaller) frequency.
The Visible Spectrum
Visible light waves are the only electromagnetic waves we can see. We see these waves as the colors of the rainbow. Each color has a different wavelength. Red has the longest wavelength and violet has the shortest wavelength. When all the waves are seen together, they make white light.
When white light shines through a prism, the white light is broken apart into the colors of the visible light spectrum. Water vapor in the atmosphere can also break apart wavelengths creating a rainbow.
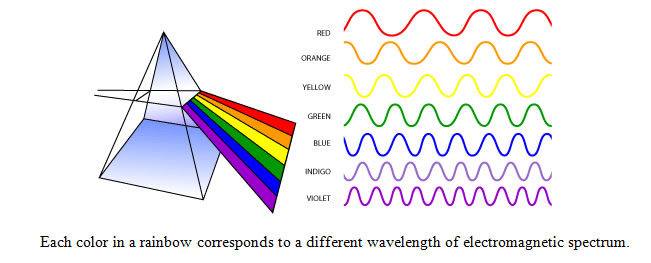
color |
|
v(*1014 Hz) |
Energy (*10-19 J) |
violet |
4000 |
7.5 |
5.0 |
indigo |
4600 |
6.5 |
4.3 |
blue |
4750 |
6.3 |
4.2 |
green |
4900 |
6.1 |
4.1 |
yellow |
5650 |
5.3 |
3.5 |
orange |
5750 |
5.2 |
3.45 |
red |
6000 |
5.0 |
3.3 |
Planck’s Equation
In Planck's assumption, radiant energy is emitted in small bursts, known as "quanta". Each of the bursts called a "quantum" has energy E that depends on the frequency f of the electromagnetic radiation by the equation:
E = hv
where h is a fundamental constant of nature, the "Planck constant". 6.626e-34 J.s
This equation is later found to be true for all EM radiant energy emitted or absorbed.

Photo of Max Planck.
Courtesy of AIP Emilio Segre Visual Archives, W.F. Meggers Collection.
Planck's equation implies the higher the frequency of a radiation, the more energetic are its quanta.
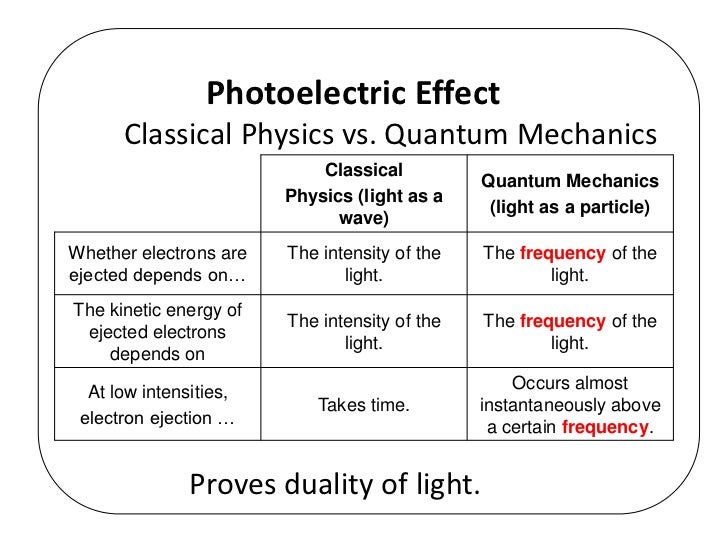
The Photoelectric Effect