CHM3120L
Introduction to Analytical Chemistry: Laboratory
| Lab Schedule |
| LON-CAPA Pre-Labs |
| Resources |
| Contact Instructor |
| Chemistry Home Page |
![]()
Determination of Ascorbic Acid by Redox Titration : Introduction
Iodine is a versatile redox reagent because its potential falls in the middle of the range of potentials observed in aqueous solutions. Thus in the presence of strong oxidants, such as dichromate, iodide is oxidized to iodine; in the presence of reducing agents, such as As (III), iodine is reduced to iodide.
Solid I2 is only slightly soluble in water, but in the presence of excess iodide it forms the soluble triiodide ion, I3-, and it is in this form that it is used for redox titrations. Reducing agents are determined by direct titration with standard I3-. For the determination of oxidizing agents it is not feasible to titrate directly with standard iodide, because a high concentration of iodide is needed to form the I3- complex. Instead excess iodide is added to oxidizing agents, and the excess I3- formed is titrated with a standard solution of a reducing agent, thiosulfate, S2O32-.
An advantage to all of these analyses is the ready availability of a specific indicator, starch. I3- reacts with starch to form an intense blue color that is visible even at very low I3- concentrations. In direct titrations with I3- the endpoint is signaled by the appearance of the blue color when the first trace of I3- is produced after the equivalence point. In titrations of triiodide with thiosulfate, the endpoint is signaled by the disappearance of the blue color. Care must be taken, however, to add the starch after most of the I3- has already reacted. In the presence of large I3- concentrations a rather stable complex forms, and the blue color persists beyond the equivalence point.
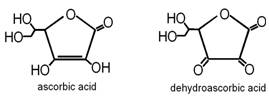
In this exercise you will determine the weight percent of ascorbic acid, Vitamin C, in Vitamin C tablets. Ascorbic acid, C6H8O6, (MW = 176.12) is oxidized by iodine, to dehydroascorbic acid, C6H6O6. The I3- will be generated in situ by adding a known volume of a standard iodate, IO3-, solution to a solution of ascorbic acid and iodide. The iodate oxidizes the iodide to form I3-, which reacts in turn with the ascorbic acid. The excess I3- is titrated with a standard solution of S2O32-. From the moles of I3- produced, calculated from the volume of standard IO3- solution added, and the moles of excess I3-, calculated from the volume of standard S2O32- used, the moles of ascorbic acid can be calculated.
The concentration of the standard iodate solution can be calculated directly from the weight of KIO3 used. The thiosulfate solution must be standardized. The standardization is carried out against the standard iodate solution. A known volume of the standard iodate solution is added to an excess of iodide, generating a known amount of I3-. The I3- is titrated with the S2O32- to a starch endpoint.
Care must be taken in preparing and storing thiosulfate solutions. Although sodium thiosulfate solutions are resistant to air oxidation, they do tend to decompose to give sulfur and hydrogen sulfite ion. Variables that influence the rate of decomposition include pH, the presence of microorganisms, the presence of Cu (II) ions, and exposure to sunlight. To minimize the need for restandardization of the thiosulfate solution, it will be prepared under reasonably sterile conditions, at a pH between 9 and 10, and stored in the dark.
Redox Titration Animation