CHM3120L
Introduction to Analytical Chemistry: Laboratory
| Lab Schedule |
| LON-CAPA Pre-Labs |
| Resources |
| Contact Instructor |
| Chemistry Home Page |
![]()
Spectrophotometric Determination of Iron in Drinking Water : Introduction
Several contaminants in drinking water can be determined spectrophotometrically, including iron. Although iron is easily determined in contaminated water containing >1 ppm (1 mg/L), federal and state regulations limit the iron content of drinking water to <1 ppm. Thus, an intensely colored complex must be formed to detect the presence of these low levels of iron spectrophotometrically. As is the case whenever trace quantities of an analyte are being measured, cleanliness of equipment, glassware, etc. is essential to prevent positive determinate errors (false positives) due to laboratory contamination.
One widely used iron complex is iron(II)-o-phenanthroline, which is orange-red and easy to detect. Like most metal complexation reactions, the metal ion must compete with H3O+ ions, and thus the metal complex will not form in strongly acidic solutions. On the other hand, most metals form insoluble metal hydroxides in basic solutions. For these reasons the iron determination using o-phenanthroline is carried out in a slightly (pH 4-6) acidic solution.
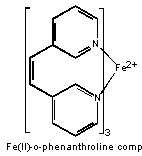
In most water samples iron exists in its oxidized form, (Fe(III)), due to the presence of oxygen. Since it is the Fe(II) species that forms the complex with o-phenanthroline, a reduction must first be carried out. This can be accomplished by the addition of hydroxylamine. In the presence of an excess of hydroxylamine, the Fe(II)-o-phenanthroline complex is quite stable.
Your unknown is representative of a contaminated water sample. It has been spiked to produce an Fe concentration greater than 1 ppm Fe.