Dynamic covalent chemistry: a new tool in the design of chemoselective radical reactions
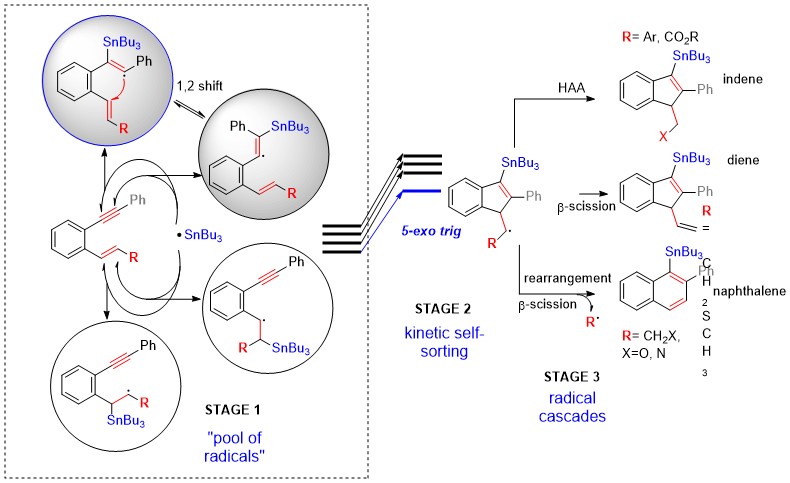
We found that remarkable chemo- and regio-selectivity of the initial radical attack in multifunctional substrates can be accomplished via the combination of Dynamic Covalent Chemistry (DCC) with kinetic self-sorting. Not only is the addition of Sn-radicals to alkenes and alkynes fast and reversible but we have also identified a new 1,2 stannyl shift as a low-barrier mechanism for the conversion of an unproductive vinyl radical to the radical adduct which can undergo fast 5-exo-trig closure (serving as a route for kinetic self-sorting). Furthermore, we have also identified substitution patterns that can selectively direct the initially formed cyclic radical to react further in one of the following three ways: H-abstraction, β-scission and, ring expansion.
Selected publications:
Alkenes as Alkyne Equivalents in Radical Cascades Terminated by Fragmentations: Overcoming Stereoelectronic Restrictions on Ring Expansions For the Preparation of Expanded Polyaromatics. Mohamed, R.; Mondal, S.; Gold, B.; Evoniuk, C. J.; Banerjee, T.; Hanson, K.; Alabugin, I. V. J. Am. Chem. Soc., 2015, 137, 6335-6349. http://pubs.acs.org/doi/abs/10.1021/jacs.5b02373.
Drawing from a pool of radicals for the design of selective enyne cyclizations. Mondal, S.; Mohamed, R. K.; Manoharan, M.; Phan, H.; Alabugin, I. V. Org. Lett., 2013, 15, 5650–5653. http://pubs.acs.org/doi/abs/10.1021/ol4028072.