Light-activated reagents for efficient double strand DNA cleavage
DNA is not only the central molecule of life but also a target for cancer therapy. If cellular DNA is damaged beyond repair, cells initiate self-programmed death, or apoptosis. If one can limit this process to cancer cells and make them will self-destruct selectively, efficient DNA damage can be used for the development of anticancer agents. Not surprisingly, natural enediyne antibiotics, molecules hailed as "the most efficient anticancer agents" known to date utilize DNA damage as is the key step in the mechanism of their biological activity.
Selectivity and efficiency are the most important questions in the design of DNA-cleaving agents for cancer therapy. How can one limit the damage to the cancerous cells without affecting the healthy tissue? How can one overpower the efficient DNA repair mechanisms which usually reverse the damage and keep our genome functioning for many years?
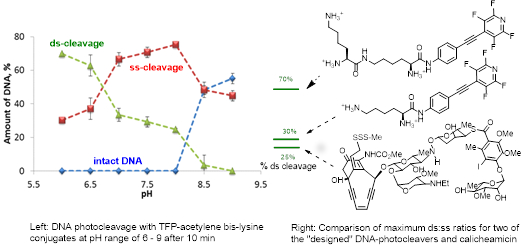
The astounding biological activity of natural enediyne antibiotics is associated with their ability to cause simultaneous damage of both DNA strands ("the doble stranded" or "ds" DNA cleavage). Although even the best of natural enediynes, calicheamicin, induces, at best, ~25-33% of the double stranded cleavage, this is still enough to account for the record-breaking biological activity of enediynes (and also set a world record for ds:ss (single-strand) DNA cleavage ratio for a non-enzymatic molecule).
Unlike the readily repairable single-strand ("ss") DNA cleavage where the two DNA strands are still kept together with hydrogen bonding, DNA with both strands broken is much more difficult "patient" for repair enzyme (the little repairman shown on the left). As the result, even moderately efficient ds DNA cleavage may lead to the highly efficient apoptosis.
Our group developed a family of light-activated molecules with the ds-DNA cleavage efficiency rivaling (for the first generation) or exceeding (for the second generation) the ds:ss ratio of calichiamicin. Not only the use of light allows us to activate our molecules exactly at the right time and right place, inside of the cancer cells but, remarkably, the high efficiency of ds-DNA cleavage is observed under slightly acidic conditions suitable for selective targeting of certain cancers. TFP-enediynes and acetylenes induce 100-1000 times more double-strand (ds) DNA cleavage than expected from a combination of random coincident single stranded cleavages. These ds:ss ratios for the DNA damage exceed that of calicheamicin, the most effective natural enediyne ds DNA-cleaver known.
Reviews:
Breiner, B.; Kaya, K.; Roy, S.; Yang, W.-Y.; Alabugin, I. V. Hybrids of Amino Acids and Acetylenic DNA-Photocleavers: Optimizing Efficiency and Selectivity for Cancer Phototherapy. Invited “Perspective”. Org. Biomol. Chem. 2012, 10, 3974-3987. http://pubs.rsc.org/en/Content/ArticleLanding/2012/OB/C2OB00052K.
Mohamed, R. K.; Peterson, P. W.; Alabugin, I. V. Concerted Reactions that Produce Diradicals and Zwitterions: Electronic, Steric, Conformational and Kinetic Control of Cycloaromatization Processes. Chem. Rev., 2013, 7089–7129. (invited article, special issue on Reactive Intermediates) http://dx.doi.org/10.1021/cr4000682.
Selected publications:
Opening Enediyne Scissors Wider: pH-Dependent DNA Photocleavage by meta-Diyne Lysine Conjugates. Kaya, K,; Johnson, M.; Alabugin, I. V. Photochemistry and Photobiology (the Michael Kasha issue), 2015, 91, 748-758. http://onlinelibrary.wiley.com/doi/10.1111/php.12412/pdf.
Yang, W.-Y.; Roy, S.; A. R.; Phrathep, B.; Rengert, Z.; Zorio, D. A. R.; Alabugin, I. V. Engineering Multiple pH-Gated Transitions for Selective and Efficient Double Strand DNA Photocleavage in Hypoxic Tumors. J. Med. Chem., 2011, 54, 8501−8516. http://pubs.acs.org/doi/abs/10.1021/jm2010282.
Yang, W.-Y.; Breiner, B.; Kovalenko, S. V.; Ben, C.; Singh, M.; LeGrand, S. N.; Sang, A. Q.-X.; Strouse, G. F., Copland, J. A.; Alabugin I. V. C-Lysine Conjugates: pH-Controlled Light-Activated Reagents for Efficient Double Stranded DNA Cleavage with Implications for Cancer Therapy, J. Am. Chem. Soc., 2009, 131, 11458–11470. http://pubs.acs.org/doi/abs/10.1021/ja902140m.
Breiner, B.; Schlatterer, J. C.; Kovalenko, S. V.; Greenbaum, N. L.; Alabugin I. V. Protected 32P-Labels in Deoxyribonucleotides: Investigation of Sequence Selectivity of DNA Photocleavage by Enediyne-, Fulvene-, and Acetylene-Lysine Conjugates. Angew. Chem. Int. Ed., 2006, 45, 3666-3670. http://www3.interscience.wiley.com/cgi-bin/fulltext/112598740/PDFSTART.
Breiner, B.; Schlatterer, J. C.; Kovalenko, S. V.; Greenbaum, N. L.; Alabugin I. V. DNA Damage-Site Recognition by Lysine Conjugates. Proc. Natl. Acad. Sci., 2007, 104, 13016-13021. http://www.pnas.org/content/104/32/13016.
Kovalenko, S. V.; Alabugin I. V. “Lysine-Enediyne Conjugates as Photochemically Triggered DNA Double-Strand Cleavage Agents”. Chem. Comm. 2005, 1444 – 1446.