Stereoelectronic control of cyclization reactions – from all-exo-cyclizations to LUMO Umpolung
The application of stereoelectronic guidelines to cyclization reactions lead to the refined rules of cyclizations that include a variety of mechanistic pathways: from usual radical and nucleophilic reactions that favor exo-cyclizations to electrophile-assisted nucleophilic closures with LUMO umpolung and photochemical cyclizations with alkene twisting that allow the less common endo-cyclizations to proceed efficiently. Each pattern shown below has its own unique reactivity/selectivity combination.
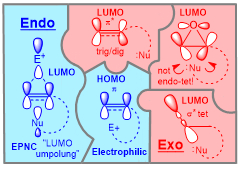
Selected publications:
Alabugin, I. V.; Gilmore, K. Chem. Commun., 2013, 49, 11246. Finding the right path: Baldwin “Rules for Ring Closure” and stereoelectronic control of cyclizations. http://pubs.rsc.org/en/content/articlehtml/2013/cc/c3cc43872d.
Alabugin, I. Gilmore, K.; Manoharan, M. Rules for Anionic and Radical Ring Closure of Alkynes. J. Am. Chem. Soc. 2011, 133, 12608-12623, http://pubs.acs.org/doi/abs/10.1021/ja203191f.