Drug formulations are heterogeneous solids comprised of one or more active pharmaceutical ingredients (APIs), along with numerous other materials known as excipients, which are non-medical ingredients that serve as stabilizers of the API, bulking agents, diluents, or therapeutic enhancers (e.g., improved solubility, adsorption, lubrication, etc.) It is often difficult to detect the API in a dosage form, due to interfering signals from the complex array of excipients.
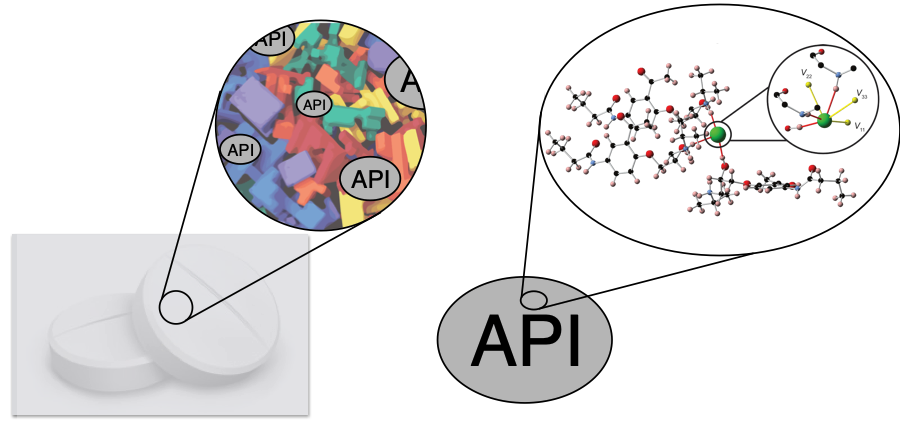
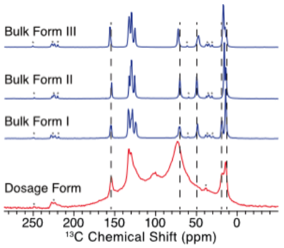
Our research group has pioneered 35Cl SSNMR an alternative approach for studying APIs. 35Cl SSNMR spectra can serve as spectral fingerprints of each solid form of an API. As such, they can be used to identify the solid phase present in a dosage form thereby providing quality assurance during drug development and manufacturing.
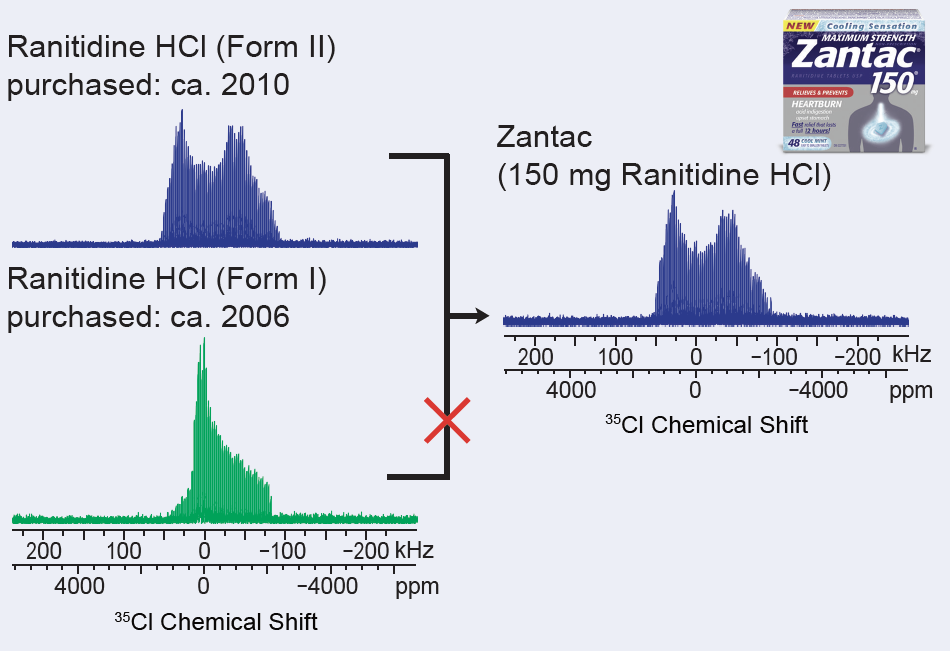
Here, 35Cl SSNMR is used to differentiate two forms of ranitidine HCl, the active ingredient in Zantac, which is an H2 (or histamine-2) blocker that is used to relieve and prevent heartburn.
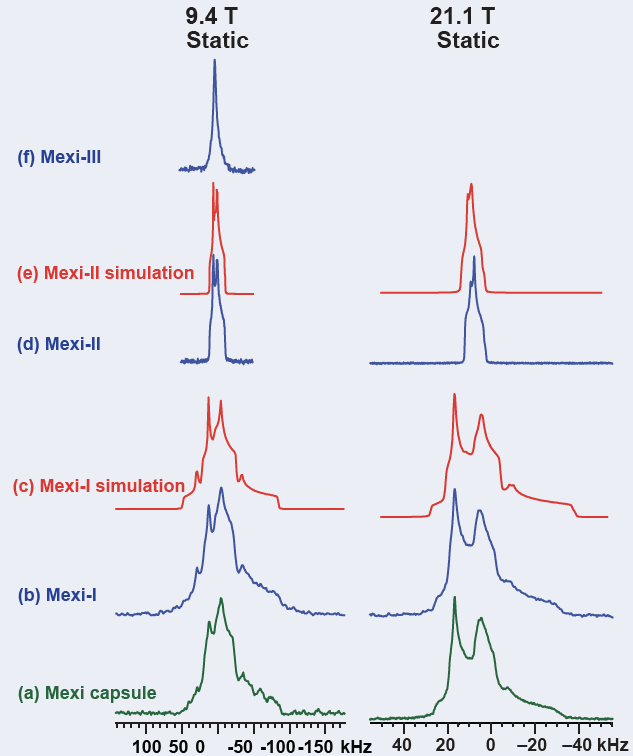
35Cl SSNMR is very useful for not only differentiating the three polymorphs of mexiletine HCl, a drug used to treat abnormal heart rhythms, chronic pain, and muscle stiffness, but also identifies mexiletine Form 1 as the polymorph present in the dosage form.
Selected pharmaceutical papers
- Holmes, S. T.; Hook, J. M.; Schurko, R. W. Nutraceuticals in Bulk and Dosage Forms: Analysis by 35 Cl and 14 N Solid-State NMR and DFT Calculations. Mol. Pharm. 2021, acs.molpharmaceut.1c00708. https://doi.org/10.1021/acs.molpharmaceut.1c00708.
- Holmes, S. T.; Vojvodin, C. S.; Schurko, R. W. Dispersion-Corrected DFT Methods for Applications in Nuclear Magnetic Resonance Crystallography. J. Phys. Chem. A 2020, 124, 10312–10323. https://doi.org/10.1021/acs.jpca.0c06372.
- Holmes, S. T.; Engl, O. G.; Srnec, M. N.; Madura, J. D.; Quiñones, R.; Harper, J. K.; Schurko, R. W.; Iuliucci, R. J. Chemical Shift Tensors of Cimetidine Form A Modeled with Density Functional Theory Calculations: Implications for NMR Crystallography. J. Phys. Chem. A 2020, 124, 3109–3119. https://doi.org/10.1021/acs.jpca.0c00421.
- Hirsh, D. A.; Holmes, S. T.; Chakravarty, P.; Peach, A. A.; Dipasquale, A. G.; Nagapudi, K.; Schurko, R. W. In Situ Characterization of Waters of Hydration in a Variable-Hydrate Active Pharmaceutical Ingredient Using 35Cl Solid-State NMR and X-Ray Diffraction. Cryst. Growth Des. 2019, 19, 7349–7362. https://doi.org/10.1021/acs.cgd.9b01218.
- Hirsh, D. A.; Su, Y.; Nie, H.; Xu, W.; Stueber, D.; Variankaval, N.; Schurko, R. W. Quantifying Disproportionation in Pharmaceutical Formulations with 35 Cl Solid-State NMR. Mol. Pharm. 2018, 15, 4038–4048. https://doi.org/10.1021/acs.molpharmaceut.8b00470.
- Peach, A. A.; Hirsh, D. A.; Holmes, S. T.; Schurko, R. W. Mechanochemical Syntheses and Cl-35 Solid-State NMR Characterization of Fluoxetine HCl Cocrystals. CrystEngComm 2018, 20, 2780–2792. https://doi.org/10.1039/c8ce00378e.
- Holmes, S. T.; Schurko, R. W. Refining Crystal Structures with Quadrupolar NMR and Dispersion-Corrected Density Functional Theory. J. Phys. Chem. C 2018, 122, 1809–1820. https://doi.org/10.1021/acs.jpcc.7b12314.
- Veinberg, S. L.; Johnston, K. E.; Jaroszewicz, M. J.; Kispal, B. M.; Mireault, C. R.; Kobayashi, T.; Pruski, M.; Schurko, R. W. Natural Abundance 14 N and 15 N Solid-State NMR of Pharmaceuticals and Their Polymorphs. Phys. Chem. Chem. Phys. 2016, 18, 17713–17730. https://doi.org/10.1039/C6CP02855A.
- Hirsh, D. A.; Rossini, A. J.; Emsley, L.; Schurko, R. W. 35Cl Dynamic Nuclear Polarization Solid-State NMR of Active Pharmaceutical Ingredients. Phys. Chem. Chem. Phys. 2016, 18, 25893–25904. https://doi.org/10.1039/c6cp04353d.
- Namespetra, A. M.; Hirsh, D. A.; Hildebrand, M. P.; Sandre, A. R.; Hamaed, H.; Rawson, J. M.; Schurko, R. W. 35Cl Solid-State NMR Spectroscopy of HCl Pharmaceuticals and Their Polymorphs in Bulk and Dosage Forms. CrystEngComm 2016, 18, 6213–6232. https://doi.org/10.1039/c6ce01069e.
- Veinberg, S. L.; Friedl, Z. W.; Harris, K. J.; O’Dell, L. A.; Schurko, R. W. Ultra-Wideline N-14 Solid-State NMR as a Method for Differentiating Polymorphs: Glycine as a Case Study. CrystEngComm 2015, 17, 5225–5236. https://doi.org/10.1039/c5ce00060b.
- Hildebrand, M.; Hamaed, H.; Namespetra, A. M.; Donohue, J. M.; Fu, R.; Hung, I.; Gan, Z.; Schurko, R. W. 35 Cl Solid-State NMR of HCl Salts of Active Pharmaceutical Ingredients: Structural Prediction, Spectral Fingerprinting and Polymorph Recognition. CrystEngComm 2014, 16, 7334–7356. https://doi.org/10.1039/C4CE00544A.
- Hamaed, H.; Pawlowski, J. M.; Cooper, B. F. T.; Fu, R.; Eichhorn, S. H.; Schurko, R. W. Application of Solid-State Cl-35 NMR to the Structural Characterization of Hydrochloride Pharmaceuticals and Their Polymorphs. J. Am. Chem. Soc. 2008, 130, 11056–11065. https://doi.org/10.1021/ja802486q.
