|
Properties of Matter Most likely you have already been introduced, either in the lecture for this class or one of your high-school science classes, to the various properties used to characterize matter. In particular, what we are referring to are chemical and physical properties. Chemical properties only become apparent during a chemical reaction. In detail, chemical properties include a variety of concepts you will encounter this semester such as electronegativity, ionization potential, bonding, and preferential oxidation state. On the other hand, a physical property is an aspect of a particular substance that can be observed without changing its chemical composition such as color and texture. Continuing, physical properties are often measurable and can be further broken down into either an extensive property, a property that is dependent upon how much of the substance is present (E.g. mass or volume), or an intensive property, which is independent of amount (E.g. temperature or density). One of the most common physical properties of matter is mass, or as some refer to as ‘weight’. Although these two terms are often used interchangeably, they do not mean the same thing. Mass is used to describe the amount of matter, or energy, that a substance contains, while weight is scientifically defined as the total gravitational force exhibited on a substance. In fact, it is this notion that explains why astronauts are ‘weightless’ in space, yet retain their mass. Another physical property that will be investigated in this experiment is volume. Like mass, the volume of a substance is an extensive property as it is also dependent upon how much of the substance is present. Density As stated above, the physical property density, is also an intensive property, meaning that a substance's density doesn't change no matter how much of that substance is present. What is interesting about density is that it provides a direct relationship between two extensive properties, mass and volume. The density of a substance is usually reported in metric units that are a combination of the normal units used for mass, i.e. grams (g) or kilograms (kg) and the normal units for volume, milliliters (mL), liters (L) or centimeters cubed (cm3).For example, the density of solids and liquids are generally reported in g/mL, while for gases it is recorded as g/L. Density Calculations Density is calculated as the mass per unit volume of a substance.
Assuming we have a 10-lbs bag of sugar which we can get 16-cups from and a 5-lbs bag which generates 8-cups, we want to know which bag will give the greater amount of sugar per cup.
Exploring the Concept of Density through Experimentation In this particular experiment you will have two tasks: 1) to determine the metal identity of two unknown metal cylinders by matching their experimentally derived densities to a list of known metal densities and 2) to create a successful ‘submarine’ by matching the density of the mock submarine (made of a balloon and metal pellets) to the density of the water in which it resides. Identifying a Metal by its Density In order to determine the density of a metal cylinder, you will need to measure two physical properties of that cylinder: its mass and its volume. Mass is simple to obtain. We simply weigh the cylinder on an analytical balance, described below. Obtaining the volume of your metal cylinders will require more work. Determining Volume To determine the volume of your cylinders, you will have to use one of two methods, volume by displacement or volume by calculation. The first method, volume by displacement, uses the displacement of water in a graduated cylinder to determine the volume of the metal cylinder:
The volume of water that is displaced by the metal cylinder is exactly equal to the volume of the metal cylinder itself.
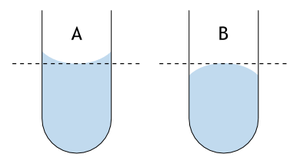
Reading a Graduated Cylinder Accurate measurement of this volume depends on the scientist's ability to correctly read the scale on the graduated cylinder. This reading is made more complicated by the formation of a meniscus caused by capillary action. Whenever liquids are held in a narrow container, the surface tension of the liquid causes a marked curvature of the upper surface. Specifically, this is referred to as a meniscus.
A: Read the bottom of a concave meniscus.
In the case of water, the meniscus is concave and in order to accurately record the volume, you have to observe where the center of the curve is located. At this point, it is appropriate to mention that in order to get the most accurate results you also have to incorporate some estimation. Graduated cylinders, thermometers, rulers, and other instruments are all read to the closest mark and then estimated to one decimal place further. This means dividing the space between marks into ten smaller marks. For example, when reading a graduated cylinder with marks for each milliliter, you should record the volume to the nearest tenth of a milliliter. It is automatically assumed that the last number reported in your data is an estimate; this is also true for instruments which provide a digital output. Volume by Calculation The second method you can use to obtain the volume of your metal cylinders is called volume by calculation. Since your metal cylinder is, in fact, cylindrical, the equation shown below, where r is the radius of your cylinder and h is its height, can be used to ascertain its volume.
The Vernier Caliper You just need to measure the radius and the height (length) of your cylinder. In order to measure these dimensions accurately, we will be using a device called a Vernier caliper.
In order to use the instrument appropriately please note the following guidelines:
Measuring Mass In addition to measuring the volume of your unknown metal cylinder, in order to determine its density, you must also find its mass. There are many types of scales and balances, varying from range and accuracy, being used in today’s laboratories and commercial operations. Different balances exist for different purposes; you would not use a milligram balance to weigh out two tons of fertilizer. All of the balances in the laboratory use the same general technique to determine the mass of an object. What follows is a brief introduction to each of the different types of balances commonly used: 1. Double-pan Balance: objects of an accurately known mass are added to one side of a two-pan scale to ‘balance’ the object to be weighed until there is no difference between the two sides.
 2. Mechanical Balance: similar to the double-pan balance, instead of placing weights on one side of the balance, internal weights inside the instrument are connected to dials on the front of the balance.
3. Electronic Balance: a magnetic field is used to observe the deflection—which is compared to stored data for known standards—of the pan when an object is placed on it.
In a career as a scientist or engineer, all of these types of balances will be encountered. It would benefit you to become familiar with the ways in which each of them operates, learn how to read the scales with the appropriate amount of precision and accuracy. The balances we use in this lab are electronic and digital. The proper use of the balance can be seen here.
Now that you have all of the information you need on the measurement and calculation of density, you are ready to begin... A Submarine Adventure Picture this…you are at war with a group of aliens, we will call “Gatorians” for lack of a better term. They look like big lizards with green skin and orange eyes. Yuck! They have taken over your world and have isolated you and 19 others on an island somewhere in the South Pacific. But you have hope. If you can rendezvous with the military in Hawaii, you and your friends will be protected. Your only problem is how to get there. The “Gatorians” patrol the ocean surface continuously, and Hawaii is hundreds of miles from your position. Then someone in your group suggests that you build a submarine. That way you can avoid the “Gatorians” and still make it to Hawaii. You all decide that a submarine is the only way to go and then begin to design it. In order for the design to work, the submarine must float off the bottom of the ocean but not break the surface. This means that the density of the sub as a whole has to be very similar to the density of the water that surrounds the island. Real submarines use ballast systems, of course, but you have limited time and supplies so a design based on density is your only option (not to mention none of you are physicists and a workable ballast system is way out of your league). The group decides that they want you (the only scientist in the group) to design the sub and to make sure it will work. Before using what little resources you have, building the actual submarine, you devise the following experiment to test the idea first. Using 20 metal pellets to represent masses of the 20 people that have to travel inside the submarine and a balloon to represent the sub itself, you calculate the density of the salt water and inflate the balloon to just the right volume so that the “submarine’s” density allows it to hover between the bottom of a pail of water and the surface. Your model system is quite a bit smaller than the sub will have to be, but if you can get the model to work then simply making the real sub 100 times bigger should also work as long as the density of the sub and the water remains the same.
|
 |
A Submarine Adventure: Density Saves the Day