SAFETY NOTES: In this and every other lab this semester you must wear long pants, closed-toed shoes, and shirts that cover your entire midriff area. You will also need goggles. Failure to comply with any of these requirements will result in you being asked to leave the laboratory.
GENERAL INSTRUCTIONS: Each Student will work as an individual for this lab.
| Part 1: Density of a Metal Cylinder | |
 |
Collect 2 unknown metal cylinders from the front counter. (One from each bin). Make close observations about the markings, color and condition of the cylinders in your lab notebook. |
 |
Using the appropriate method, volume by displacement or volume by measurement, record the measurements of the cylinders and calculate their volumes. |
 |
Take the cylinders into the balance room and weigh them using standard weighing technique. Be sure to wipe them down with Kimwipes to remove any fingerprints, etc. Record both masses in your lab notebook. |
| Part II: Building a Submarine | |
 |
Take out your 10 mL graduated cylinder from your drawer. Take it to the balance room and weigh it using standard weighing technique. Be sure to wipe it down with Kimwipes to remove any fingerprints, etc. Record the mass in your lab notebook. |
 |
Using the same 10 mL graduated cylinder, collect ~1-4 mL of "ocean" water from the reservior on your lab bench. Record the exact volume to the 0.01 mL in your lab notebook. |
 |
Take the graduated cylinder with the water in it to the balance room and weigh it using standard weighing technique. Be sure to wipe it down with Kimwipes to remove any fingerprints, etc. Record the total mass in your lab notebook. |
 |
Collect a balloon and 20 metal pellets from the front counter. Take the balloon and pellets to the balance room and weigh them using standard weighing technique. The balances will only weigh ~100 g so you may need to weigh the pellets in groups and sum the total weight. Be sure to wipe them down with Kimwipes to remove any fingerprints, etc. Record the mass(es) in your lab notebook. |
 |
Carefully place the pellets inside the balloon. |
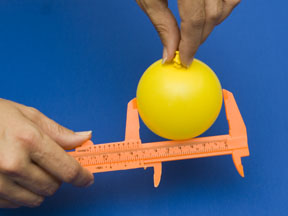 |
Calculate the density of the "Ocean" water and using the equation given here calculate the diameter of the balloon needed to equal that density. Blow up your balloon to this diameter and seal it. |
 |
Test the viability of your "submarine" by placing it in the "Ocean". A good submarine will neither sink nor float but rather hover just below the surface. Make notes in your lab notebook describing the results of this test.
|

