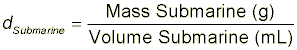|
The purpose should be several well constructed sentences that cover the topics that this experiment is designed to cover. This can include both concepts and experimental techniques. The procedure section should reference the lab manual and note any changes that were made to the procedure while performing the experiment. Now that the lab manual is online there is a slightly different method of citation: In MLA style, the components are arranged this way:
Make sure to note which method you used when the procedure gives you a choice. The data section should include all observations made during lab of the cylinders, balloons and anything else involved in the experiment. A data table for determining the density of a metal cylinder should also be included with the following information a) the measurements of the cylinders, b) the volume of both cylinders, c) mass of the cylinders d) the density of the cylinders and e) identity of unknown metals. A second data table with the submarine data should be included. Be sure that the table includes the following information a) mass of graduated cylinder, b) volume of ocean water, c) mass of water, d) weight of balloon with weights, e) density of ocean water, f) volume of balloon needed to match salt water density, g) final volume of submarine design and h) final density of submarine design. The majority of the values needed to complete the data section were recorded in lab. There are a few calculations that must be done though. The volume of the cylinders can be determined by one of these formulas depending on which methods you used. For volume by displacement:
For volume by measurement:
The density of the cylinders can be determined using the formula Density = mass/ volume. The units of density should be g/mL. For the second data table the mass of the water can be determined by subtracting the mass of the empty graduated cylinder from the mass of the graduated cylinder with water in it . The density of the ocean water and the balloon volume needed to match this density can be determined using the following formulas. If you need additional help with these calculations see the procedure.
The final volume of the submarine is determined using:
The final density of submarine is determined using:
The calculations section should have example calculations of the following: a) the volume of the cylinders, b) mass of water, c) density of ocean water, d) volume of balloon needed to match salt water density, e) radius of the balloon, f) final volume of submarine and g) final density of submarine. The conclusion section should be in paragraph format. This section should report the information in the data section. For the density of the metal cylinder section be sure to report the density and identity of the unknown metals. Discuss any differences in the known and unknown density and the percent error. Also include any experimental errors that might have led to these differences. For the submarine data report the density of the ocean water, the ideal volume of the submarine and the final volume and density of the submarine. Discuss the trial and error portion of making the submarine and the differences in the ideal volume of the submarine and the final volume of the submarine. Finally discuss any errors in the submarine design and the experiment. Answer the following Questions: 1) Give an example of the use of density (other than submarines) that you have observed in your own life. 2) What is third person neutral and why is it used in the writing of scientific papers? |
 |
A Submarine Adventure: Density Saves the Day