Sustainability: Microwave Reactors for Synthesis

Microwaves have been used for decades to heat up our food, but they can also be used to drive chemical reactions. The sustainability of reactions performed in a mircowave reactor is achieved by specific heating of microwave active (dipole containing) molecules and metals, which allows for increased heating efficiency of the target species and reduced need for energy input to heat the surrounding solvent, thus making microwave reactions rapid, efficent and high throughput.
This method of synthesis is further enhanced by introducing the use of an autosampler. The Explorer autosampler allows for up to 24 different reactions to be setup to run automatically, making it possible to run multiple samples quickly under the same conditions or each sample under different conditions programmed by the user.
Understanding the physical mechanisms by which microwave heating takes place is of key importance to designing and utilizing microwave energy for commercial applications.
Sustainability: Supercritical Carbon Dioxide Reactor
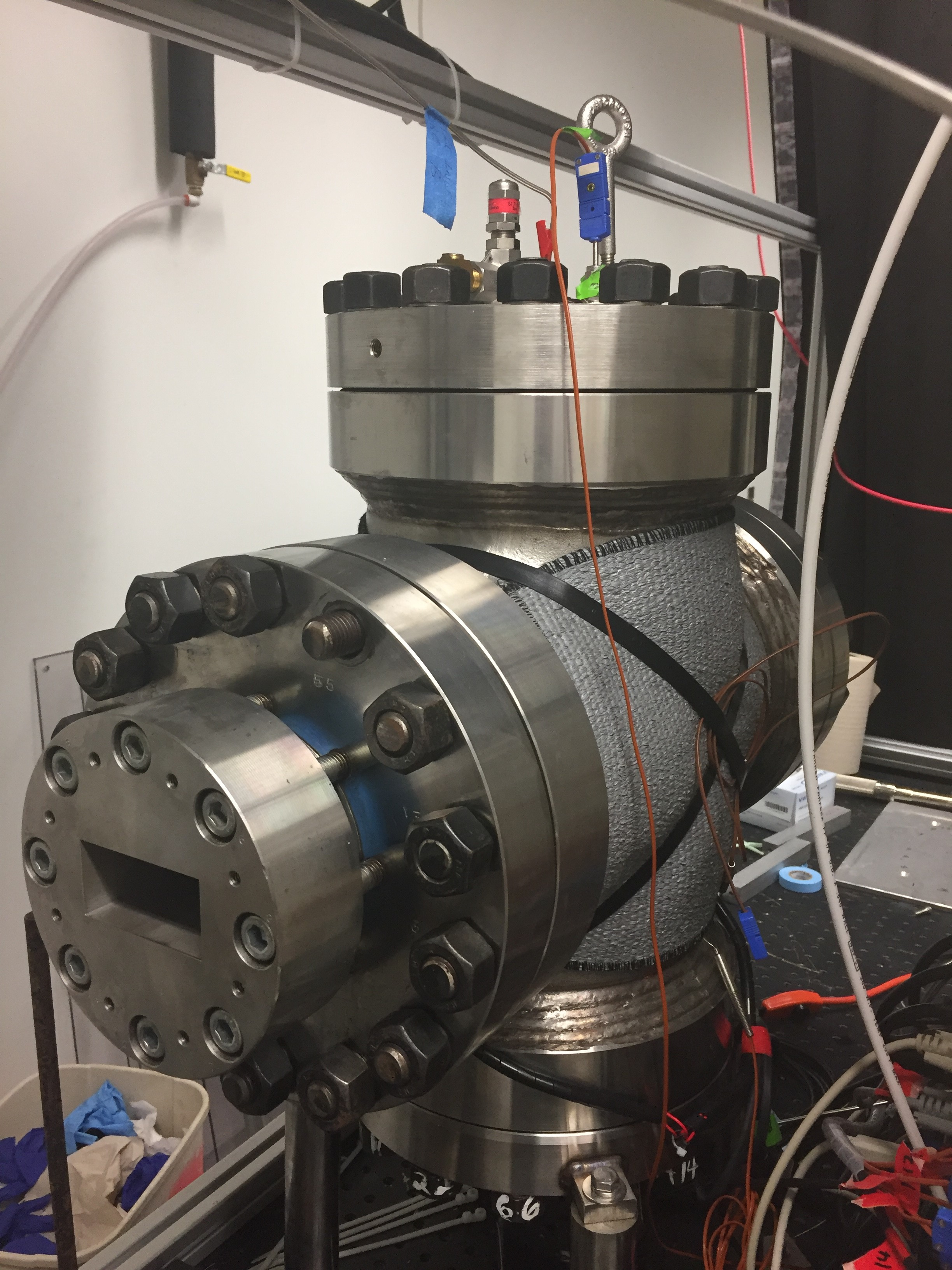
The supercritical carbon dioxide microwave reactor (scCO2 MW reactor) was designed by Dr. Jeffery Owen’s Materials Group at Tyndall AFB. This reactor consists of a supercritical CO2 chamber with microwave transparent windows and coupled with waveguide and a magnetron. It was designed for exploring microwave synthesis in a green solvent to make sustainable materials for various applications, including spinel phosphors for solid state lighting and nano magnets to replace magnetic materials based on critical elements (rare earths).
Sustainability: Understanding Microwave Heating
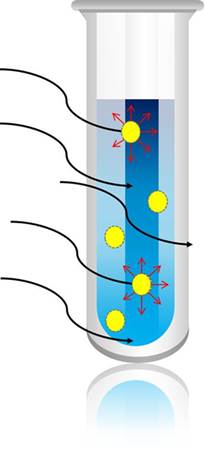
Investigating how microwaves heat materials helps us understand how we can optimize chemical reactions for use in a microwave. This is accomplished by determining each component's microwave heating property, known as the tan δ. Selective heating is defined as the disproportionate heating of a molecule over another due to one molecule having a greater tan δ. Since molecules can only absorb the whole photon, there is a greater statistical probability that a higher tan δ molecule will absorb a given photon. This can, theoretically, lead to localized areas of much higher temperature than what the bulk temperature reads, and can have the effect of increasing the reaction rate over what the bulk temperature would indicate.
We also study how the tan δ effects the way bulk materials absorb electromagnetic radiation over a broad range of communication frequencies, to design better electromagnetic shielding.
Back to Research