Area 1: Design, synthesis and characterization of inorganic nanocrystals
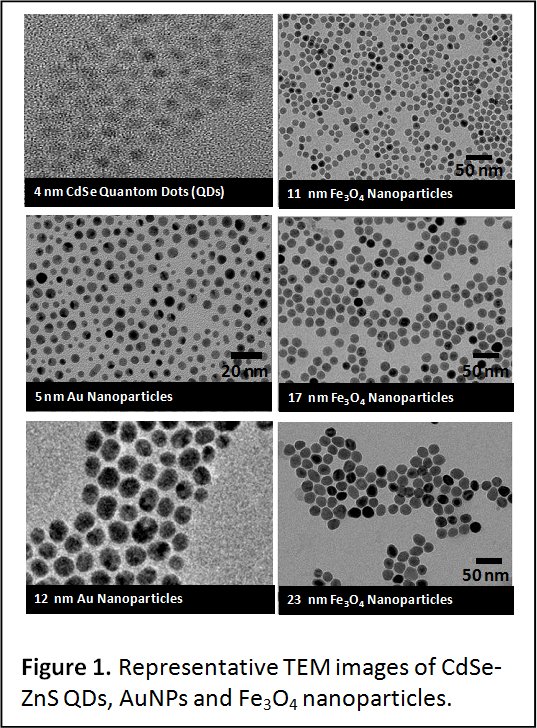
We focus on developing new synthetic schemes to prepare an array of nanocrystals made of semiconductor (quantum dots, QDs), metallic gold and silver nanoparticles and clusters, and magnetic nanocrystals, with control over their size and composition. Synthesis often involves reacting organometallic precursors at high (or room) temperature. Characterization of the structure, physical, chemical and spectroscopic properties of these materials is carried out using techniques such as UV-Vis absorption and fluorescence spectroscopy, small and wide angle x-ray scattering and diffraction, and Transmission electron microscopy, TEM (see Figures 1 & 2).
High Temperature Growth of Quantum Dots, Metal and Metal Oxide Nanoparticles. This route relies on the reduction of organometallic precursors at high temperature and in the presence of coordinating solutions. It is applicable to a wide array of materials ranging from semiconductor quantum dots and magnetic nanocrystals, to gold and other metal nanoparticles. One of the key advantages of this growth route is its ability to reproducibly provide homogeneous materials with crystalline cores and low size dispersity. Using this scheme, we have prepared high quality CdSe QDs, with low size dispersity and high fluorescence quantum yields (Figure 2). The synthesis involves the high temperature reduction (or hot injection) of cadmium and trioctyl phosphine-selenide (TOP:Se) precursors in a hot coordinating solution of trioctylphosphine and trioctylphosphine oxide (TOP/TOPO). High quality iron oxide nanocrystals with control over the size and size dispersity have also been obtained using high-temperature decomposition of iron precursors using mixtures of organic solvents and surfactants. This route has provided hydrophobic dispersions of homogeneous iron oxide nanocrystals with tunable size, crystalline cores and low size distribution (Figure 1).
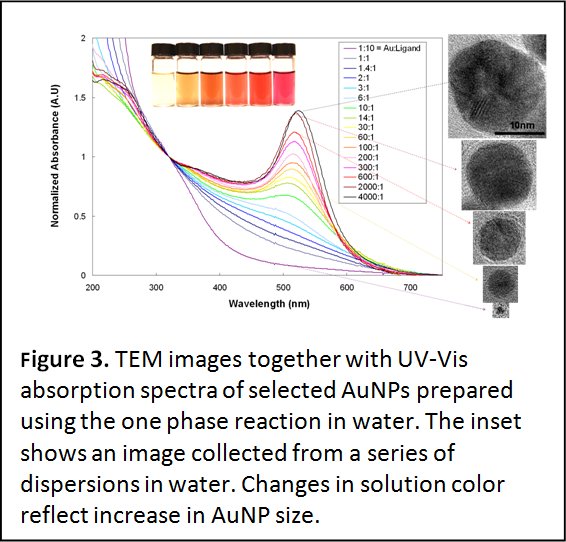
One-Phase Synthesis of Water-Soluble Gold Nanoparticles with Control over Size and Surface Functionalities. In a recent study we have reported an effective synthetic method to prepare gold nanoparticles (AuNPs) in a single aqueous phase using HAuCl4 and poly(ethylene glycol) (PEG) ligands appended with bidentate anchoring groups, namely lipoic (or thiotic) acid, which we have designed for promoting the transfer of ZnS-overcoated QDs to buffer media (see area 2). This synthetic scheme has provided nanocrystals over a broad size range, between 1.5 and 20 nm, and with narrow size distribution. The NP size was simply controlled by varying the molar ratios of Au precursor to PEG ligands (Figure 3). Further passivation of the as-prepared AuNPs permitted in-situ functionalization of the NP surface with the desired reactive terminal groups. In addition, we demonstrated that the prepared AuNPs exhibit remarkable stability in the presence of high salt concentrations, over a wide range of pHs (2-13), and a strong resistance to competition from dithiothreitol (DTT). These results are a clear manifestation of the advantages offered by our synthetic approach, where modular, multifunctional ligands presenting strong anchoring groups and hydrophilic PEG chains are used. (see Langmuir 2010, 16, 7604–7613. DOI: 10.1021/la904438s). This one-phase growth has further been expanded to using lipoic acid-zwitterion ligands, LA-ZW, (instead of LA-PEG). Indeed, we found that varying size LA-ZW-capped AuNPs, with continuous progression in the absorption features, similar to those reported using LA-PEG-OCH3, can be prepared (see ACS Nano 2013, 7, 2509–2521).
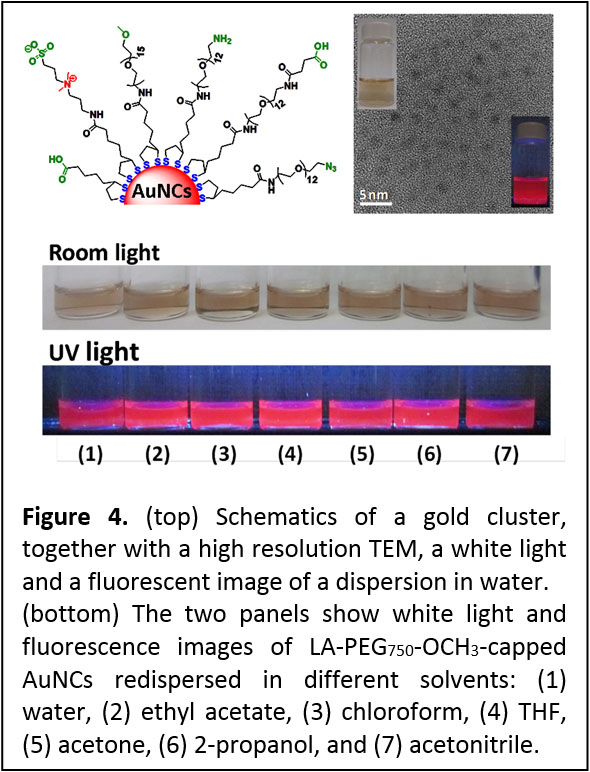
Growth of Fluorescent Polyethylene Glycol- and Zwitterion Gold Clusters. We have prepared and characterized a new set of highly fluorescent gold nanoclusters (AuNCs) using one-step aqueous reduction of gold precursor in the presence of bidentate ligands made of lipoic acid-anchoring group, appended with either a poly(ethylene glycol) short chain or a zwitterion group (Figure 4). The AuNCs fluoresce in the red to near-infrared region of the optical spectrum with emission centered at ~ 750 nm and a quantum yield (QY) of ~ 10-14 %, and they exhibit long fluorescence lifetime (up to ~ 300 ns). Dispersions of these AuNCs exhibit great long term colloidal stability, over a wide range of pHs (2-13), in the presence of high electrolyte concentrations, and a strong resistance to reducing agents such as glutathione (GSH). The growth strategy further permitted the controlled, in-situ functionalization of the NCs with reactive groups (e.g., carboxylic acid or amine), making these nanoclusters compatible with common and simple-to-implement coupling strategies, such as carbodiimide chemistry. (see ACS Nano 2013, 7, 2509-2521. DOI: 10.1021/nn30585).
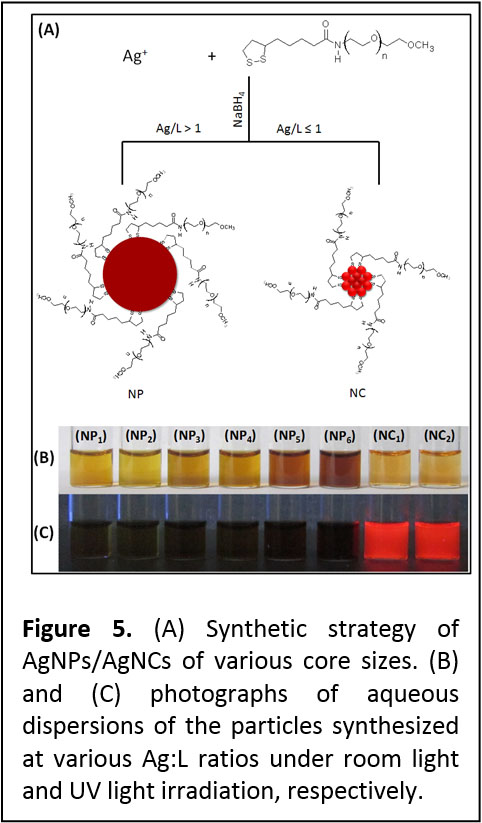
Growth of in situ Functionalized Nanoparticles and Luminescent Nanoclusters of Silver. We have expanded the above one phase growth reaction to prepare a series of silver nanoparticles (NPs) and luminescent nanoclusters (NCs) using sodium borohydride reduction of silver nitrate in the presence of molecular scale ligands made of polyethylene glycol (PEG) appended with lipoic acid (LA) groups at one end and reactive (-COOH/-NH2) or inert (-OCH3) functional groups at the other end (Figure 5). The particle size and properties were primarily controlled by varying the Ag-to-ligand (Ag:L) molar ratios and the molar amount of NaBH4 used. We found that while higher Ag:L ratios produced NPs, luminescent NCs were formed at lower ratios. We also found that non-luminescent NPs can be converted into luminescent clusters, via a process referred to as "size focusing," in the presence of added excess ligands and reducing agent. The nanoclusters emit in the far red region of the optical spectrum with a quantum yield of ~ 12%. They can be re-dispersed in a number of solvents with varying polarity while maintaining their optical and spectroscopic properties. Our synthetic protocol also allowed control over the number and type of reactive functional groups per nanocluster (see ACS Nano 2012, 6, 8950–8961. DOI: 10.1021/nn302954n).
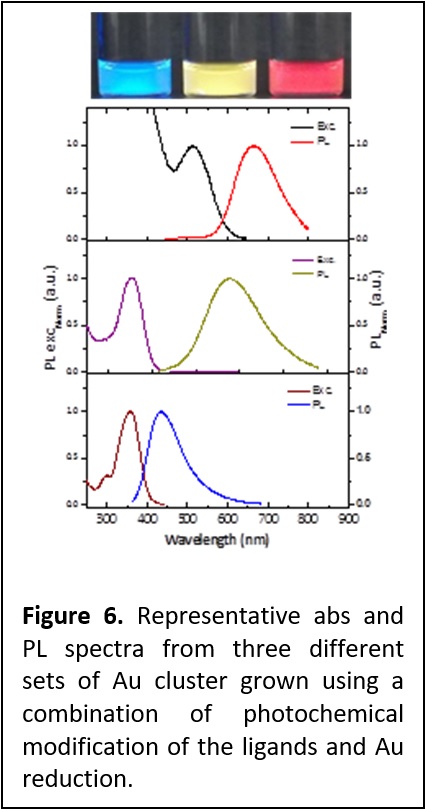
Aqueous Growth of Gold Clusters with Tunable Emission: Fluorescent gold nanoclusters (AuNCs) with tunable emission in the visible spectrum were prepared in aqueous medium using LA-PEG ligands (Figure 6). Unlike earlier methods, this method does not require additional reducing agent, such as sodium borohydride. Instead, we rely on the slow reduction of the gold precursor induced by the thiols and other sulfurous byproducts generated by the UV-irradiation of LA-based ligands. Systematic study of the influence of various experimental conditions, including the photo-irradiation time, gold precursor-to-ligand molar ratios, time of reaction, temperature and the medium pH on the growth of AuNCs showed that the reaction occurs only in basic pH in presence of excess ligand. Higher temperature also favors the reaction. Depending on the time of the reaction and nature of ligands used, the emission can be varied from yellow to red or yellow to blue. Hydrodynamic sizes were also estimated using NMR spectroscopy. The hydrodynamic sizes (RH) obtained by DOSY-NMR indicate that the size of these clusters follows the trend anticipated from the absorption and PL data, with: RH(red) > RH(yellow) > RH(blue). (Langmuir 2016, 32, 6445-6458. DOI: 10.1021/acs.langmuir.6b00950).